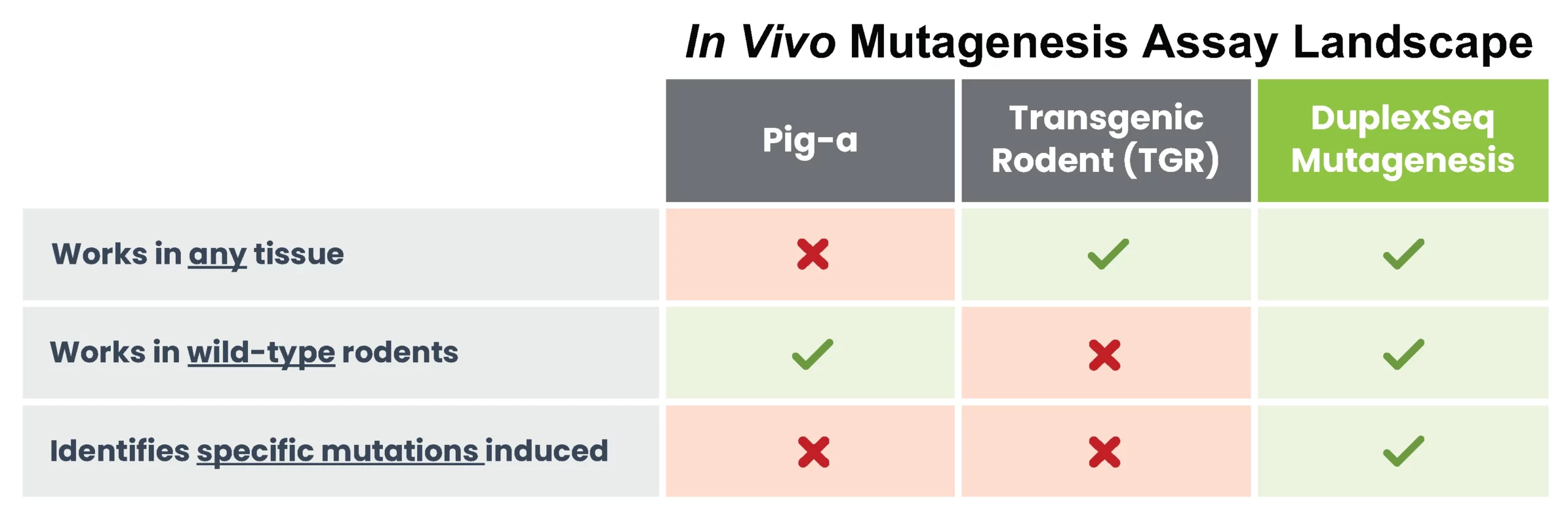
You can run DuplexSeqTM Mutagenesis Assays on commonly used tissues from transgenic or wild-type rodent studies. TwinStrand does not run animal studies – all in vivo DuplexSeq mutagenicity studies use repurposed tissues generated by partner labs.
Numerous in vivo DuplexSeq Mutagenesis studies have been published by our clients and collaborators. Here is a selection of publications from groups that are applying DuplexSeq Mutagenesis assays for in vivo testing, including bridging studies to the transgenic rodent and Pig-a assays.
For Research Use Only. Not for use in diagnostic procedures.
©2024 TwinStrand Biosciences, Inc. All rights reserved. All trademarks are the property of TwinStrand Biosciences, Inc. or their respective owners. Legal Notices
Stay up to date
Danielle LeBlanc is a Biologist at Health Canada under the supervision of Dr. Francesco Marchetti. Danielle completed her undergraduate and Masters work at Carleton University in Ottawa. Danielle is currently focusing on the implementation of Duplex Sequencing for in vivo mutagenesis assessment at Health Canada.
In her spare time, she loves to cross-country ski, bake cupcakes and craft cocktails.
Dr. Francesco Marchetti is a Senior Research Scientist at Health Canada and Adjunct Research Professor at Carleton University. He chairs the Germ Cell workgroup of the Health and Environmental Science Institute’s Genetic Toxicology Technical Committee and is a member of the Organisation for the Economic Co-Operation and Development Expert Group on Genotoxicity Testing. Dr. Marchetti has authored over 125 peer-reviewed publications.
He was Editor-In-Chief of Environmental and Molecular Mutagenesis (EMM) during 2012-2016 and serves on the editorial boards of EMM and Mutagenesis. Dr. Marchetti is the current President of the Environmental Mutagenesis and Genomic Society.