TwinStrand’s DuplexSeq™ Mutagenesis Assay is now owned and operated by Scantox. Learn more about DuplexSeq™ Mutagenesis Assays at Scantox.
Read more about the acquisition in the news.
TwinStrand Duplex Sequencing® technology reduces your sequencing error rates from 1-in-100 to 1-in-10 million, revealing data otherwise hidden. Using a combination of proprietary biochemistry and cloud-based informatics, the limitations of standard sequencing are overcome by independently tracking both strands of individual DNA molecules and comparing the results to eliminate errors.
Whether you are studying measurable residual disease, mutation signatures, cellular immunotherapy monitoring, or any low-level variants, you need accuracy beyond standard NGS. TwinStrand Duplex Sequencing reveals important low frequency variants that aren’t detectable by other methods. Get started with an off-the-shelf kit, or collaborate with us on a custom kit for your specific needs.
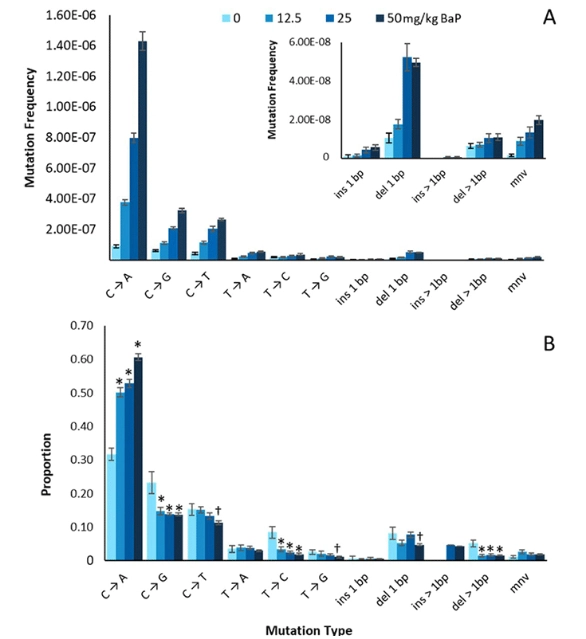
Duplex Sequencing identifies genomic features that determine susceptibility to benzo(a)pyrene-induced in vivo mutations
Researchers from Health Canada recently published a paper revealing a positive correlation between Duplex Sequencing (DS) technology and the current “gold-standard” transgenic rodent (TGR) assay to assess mutagenicity. The inter-laboratory validation study indicated consistent results across laboratories (Health Canada and TwinStrand Biosciences). The findings in this study show that DS yields novel insights into the mutagenic mode of action, which may help to overcome limitations of current mutagenicity assays for future regulatory decision making.

For Research Use Only. Not for use in diagnostic procedures.
©2026 TwinStrand Biosciences, Inc. All rights reserved. All trademarks are the property of TwinStrand Biosciences, Inc. or their respective owners. Legal Notices
Stay up to date
Danielle LeBlanc is a Biologist at Health Canada under the supervision of Dr. Francesco Marchetti. Danielle completed her undergraduate and Masters work at Carleton University in Ottawa. Danielle is currently focusing on the implementation of Duplex Sequencing for in vivo mutagenesis assessment at Health Canada.
In her spare time, she loves to cross-country ski, bake cupcakes and craft cocktails.
Dr. Francesco Marchetti is a Senior Research Scientist at Health Canada and Adjunct Research Professor at Carleton University. He chairs the Germ Cell workgroup of the Health and Environmental Science Institute’s Genetic Toxicology Technical Committee and is a member of the Organisation for the Economic Co-Operation and Development Expert Group on Genotoxicity Testing. Dr. Marchetti has authored over 125 peer-reviewed publications.
He was Editor-In-Chief of Environmental and Molecular Mutagenesis (EMM) during 2012-2016 and serves on the editorial boards of EMM and Mutagenesis. Dr. Marchetti is the current President of the Environmental Mutagenesis and Genomic Society.